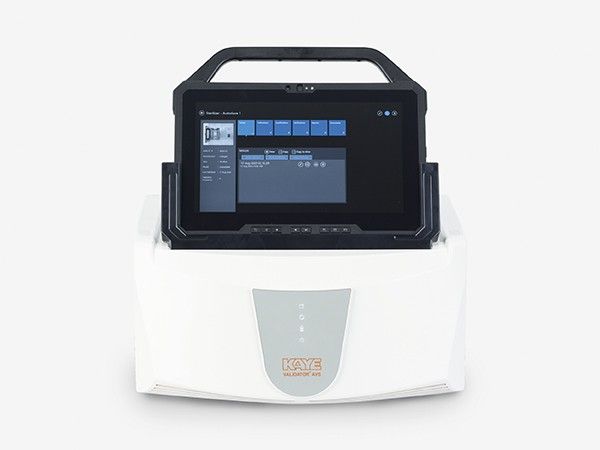
In industries such as pharmaceuticals, biotechnology, and healthcare, maintaining strict sterilization standards is non-negotiable. One of the most crucial processes in achieving this is autoclave temperature mapping. This procedure ensures uniform heat distribution within an autoclave, which is vital for the effective sterilization of instruments, equipment, or media. Without this validation, there’s a risk of contamination that can compromise safety and product integrity.
What Is Autoclave Temperature Mapping?
Autoclave temperature mapping involves placing calibrated temperature sensors or data loggers in strategic locations within an autoclave chamber during a sterilization cycle. These sensors record the temperature over time to verify whether the heat is evenly distributed throughout the chamber. The process helps identify cold spots or areas that do not reach the required sterilization temperature.
This mapping is particularly important for compliance with Good Manufacturing Practices (GMP), FDA regulations, and ISO standards. Regulatory authorities require proof that sterilization equipment consistently meets specific performance criteria, and temperature mapping provides that evidence.
Why Is Temperature Mapping Crucial?
-
Ensures Consistent Sterilization
Sterilization is only effective if all areas within the autoclave reach the validated temperature for the necessary duration. Autoclave temperature mapping helps ensure this consistency by identifying any areas that may be under-heated. -
Validates Equipment Performance
Over time, autoclave performance can drift due to wear and tear, improper loading, or calibration issues. Routine temperature mapping validates that the equipment is still operating within its required parameters. -
Enhances Quality Control
Temperature mapping contributes to overall quality control by ensuring every sterilization cycle meets defined criteria. This consistency helps avoid costly product recalls, contamination issues, and regulatory non-compliance. -
Supports Regulatory Compliance
Documentation of autoclave temperature mapping is a key requirement in audits and inspections. Demonstrating a thorough and routine mapping process shows commitment to safety and quality.
Best Practices for Effective Autoclave Temperature Mapping
-
Use Multiple Sensors: Placing sensors at various points—such as corners, center, top, and bottom—ensures a complete view of the chamber’s thermal performance.
-
Perform Mapping Annually or When Reconfiguring: Autoclaves should be mapped at regular intervals or after any significant changes to equipment or sterilization loads.
-
Analyze Data Thoroughly: Post-mapping analysis should highlight any deviations and help optimize sterilization cycles.
-
Calibrate Sensors: Ensure all temperature sensors used for mapping are accurately calibrated for reliable results.
Final Thoughts
In critical industries where sterilization is a foundational pillar of safety, autoclave temperature mapping is more than just a regulatory requirement—it’s a safeguard against potential risks. Regularly conducting and documenting this process ensures your equipment is performing effectively and your products remain uncontaminated.
Whether you’re setting up a new autoclave or validating an existing one, investing time in proper temperature mapping is a small step that offers immense returns in safety, compliance, and peace of mind.